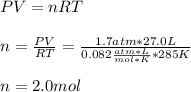Chemistry, 14.07.2020 19:01 webbhlharryteach
A cylinder contains 27.0 L of oxygen gas at a pressure of 1.7 atm and a temperature of 285 K . Part A How much gas (in moles) is in the cylinder? Express your answer using two significant figures.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:00
An excess of lithium oxide undergoes a synthesis reaction with water to produce lithium hydroxide li2o+h2o→2lioh if 1.05 g of water reacted, what is the theoretical yield of lithium hydroxide? a) 5.83 x 10–2 g lioh b) 1.17 x 10–1 g lioh c) 2.79 x 100 g lioh d) 1.40 x 100 g lioh
Answers: 1

Chemistry, 22.06.2019 10:30
Which of these is not an example of chemical weathering? a. iron-rich mineral rusting b. feldspar turning into clay c. limestone reacting with acid d. granite breaking up into sand
Answers: 1

Chemistry, 22.06.2019 12:40
Quiz1. which physical state of nitrogen has the highest entropy? a solid© b gasoc liquid
Answers: 1

Chemistry, 22.06.2019 14:50
Complete the following statements to describe solids, liquids, and gases. select the correct answer from each drop-down menu. a solid a definite volume and a definite shape. a liquid a definite volume and a definite shape. a gas a definite volume and a definite shape
Answers: 1
You know the right answer?
A cylinder contains 27.0 L of oxygen gas at a pressure of 1.7 atm and a temperature of 285 K . Part...
Questions


Engineering, 04.09.2020 18:01

History, 04.09.2020 18:01


Mathematics, 04.09.2020 18:01

Health, 04.09.2020 18:01

Mathematics, 04.09.2020 18:01


History, 04.09.2020 18:01


Mathematics, 04.09.2020 18:01





Mathematics, 04.09.2020 18:01



Computers and Technology, 04.09.2020 18:01