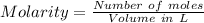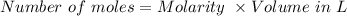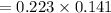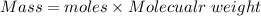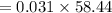Chemistry, 13.07.2020 22:01 cameronbeaugh
Suppose you need to prepare 141.9 mL of a 0.223 M aqueous solution of NaCl. What mass of NaCl do you need to use to make the solution?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 15:00
Which are forms of frozen water? check all that apply. dew frost hail rain sleet
Answers: 2

Chemistry, 22.06.2019 17:10
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2

Chemistry, 22.06.2019 23:00
What is a substance? a. a physical property of matter b. a chemical property of matter c. an element or compound that cannot be physically separated d. characteristics used to tell the difference between mixtures
Answers: 1

Chemistry, 23.06.2019 00:40
To prevent the presence of air, noble gases are placed over highly reactive chemicals to act as inert "blanketing" gases. a chemical engineer places a mixture of noble gases consisting of 4.37 g of he, 13.36 g of ne, and 36.65 g of kr in a piston-cylinder assembly at stp. calculate the partial pressure in torr of kr.
Answers: 1
You know the right answer?
Suppose you need to prepare 141.9 mL of a 0.223 M aqueous solution of NaCl. What mass of NaCl do you...
Questions

Physics, 18.01.2021 06:20


Geography, 18.01.2021 06:20

Mathematics, 18.01.2021 06:20


Mathematics, 18.01.2021 06:20







English, 18.01.2021 06:30

Biology, 18.01.2021 06:30

Mathematics, 18.01.2021 06:30

Computers and Technology, 18.01.2021 06:30


Geography, 18.01.2021 06:30

Mathematics, 18.01.2021 06:30