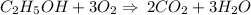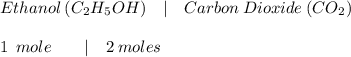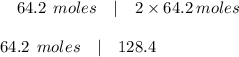Chemistry, 13.07.2020 23:01 shealynh52
Which is the number of moles of carbon dioxide produced from the complete combustion of 64.7 moles of
ethanol?
C2H60 + O2 → CO2 + H2O
Select one:
O a. 129
O b. 194
O c. 32.4
O d. 259
I AM BEGGING PLEASE HELP ME OUT

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:00
Which element in the third period would you expect to have the larger atomic radius, sodium (na) or sulfur (s)? a. sodium, because it has a higher effective nuclear charge attracting electrons in fewer energy levels. b. sodium, because it has fewer protons attracting electrons in the same energy levels. c. sulfur, because it has more protons attracting electrons in more energy levels. d. sulfur, because it has a higher effective nuclear charge attracting electrons in the same energy levels.
Answers: 2

Chemistry, 22.06.2019 19:20
15. which of the following is not human-caused groundwater pollution? a. water in an aquifer dissolves elements such as arsenic and mercury from surrounding rock. b. water in an aquifer is contaminated by leachate that seeps into the ground from a landfill. c. water in an aquifer becomes polluted with chemicals used in hydraulic fracturing, or fracking. d. water in an aquifer absorbs harmful bacteria from the drainage field of a septic tank.
Answers: 1

Chemistry, 23.06.2019 00:10
Covalent compounds: mastery test select the correct answer what is formed when atoms join together with a covalent bond? a. an ion b. a molecule c. a neutral atom d. a noble gas
Answers: 3

Chemistry, 23.06.2019 09:00
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate according to the following equation:
Answers: 2
You know the right answer?
Which is the number of moles of carbon dioxide produced from the complete combustion of 64.7 moles o...
Questions

Mathematics, 18.02.2021 04:50

Mathematics, 18.02.2021 04:50




Mathematics, 18.02.2021 04:50


Mathematics, 18.02.2021 04:50

Social Studies, 18.02.2021 04:50

Mathematics, 18.02.2021 04:50

Biology, 18.02.2021 04:50

Mathematics, 18.02.2021 04:50

History, 18.02.2021 04:50


Mathematics, 18.02.2021 04:50

Spanish, 18.02.2021 04:50

Law, 18.02.2021 04:50

Geography, 18.02.2021 04:50


Social Studies, 18.02.2021 04:50