
Chemistry, 13.07.2020 23:01 jeffhuffle17
The nature of zinc powder and Cobalt (II )oxide is heated the following reaction occurs ;
Zn(s)+CoO(s) => ZnO(s)+Co(s)
1)which metal is high in the reactivity series
2)the zink can be described as a reducing agent. using this Example explain what is the mean by the term ' reducing agent '
plz help!!!

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
24 points and brainliest to anyone who can answer under 10 minutes with best ! the table below shows the role of different substances during photosynthesis. substance role during photosynthesis glucose stores chemical energy water combines with glucose to form carbon dioxide chlorophyll traps sunlight which of the following statements would correct one of the roles listed in the table? glucose combines with carbon to form water. chlorophyll reacts with light to produce carbon dioxide. water combines with carbon dioxide during photosynthesis. chlorophyll stores chemical energy needed for photosynthesis.
Answers: 1

Chemistry, 22.06.2019 06:30
The minerals found in bones are deposited by living cells called
Answers: 1


Chemistry, 22.06.2019 17:30
A650 ml sodium bromine solution has a bromide ion concentration of 0.245 m. what is the mass (g) of sodium bromide in solution? a) 103.b)0.00155.c)16400.d) 16.4.e) 0.159
Answers: 2
You know the right answer?
The nature of zinc powder and Cobalt (II )oxide is heated the following reaction occurs ;
Zn(s)+CoO...
Questions








Physics, 02.08.2019 04:50




Mathematics, 02.08.2019 04:50





History, 02.08.2019 04:50

English, 02.08.2019 04:50

Mathematics, 02.08.2019 04:50

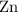 .
.

