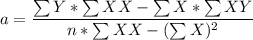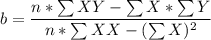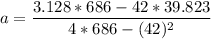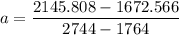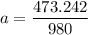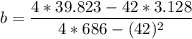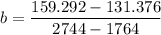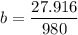Chemistry, 13.07.2020 23:01 Cheesygodxx
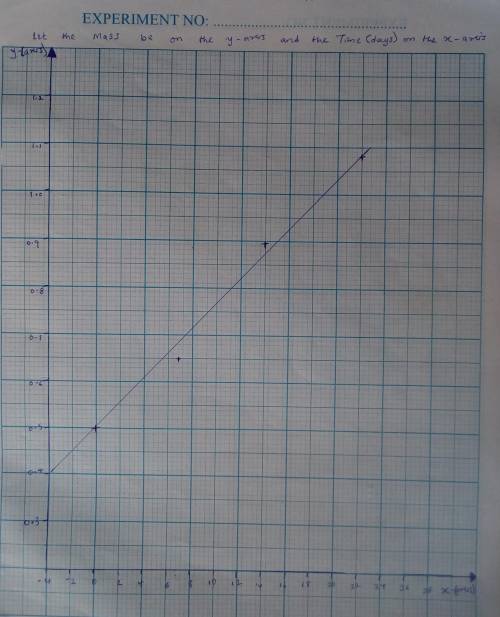
A seed of CuSO4.5H20 with a mass of 0.500 g was carefully placed into a saturated solution of copper (II) sulfate. After 7 days the mass of the seed crystal was determined to be 0.648 g. After 14 days the mass of the crystal increased to 0.899 g and after 21 days the mass of the crystal was found to be 1.081 g. Make a plot of mass vs time (days) and extrapolate to predict what would be the mass of the crystal in 28 days if the growth is linear. Include labels and units on each axis.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:10
Starch and are common polysaccharide carbohydrates found in plants. sucrose glycogen fructose cellulose
Answers: 3

Chemistry, 22.06.2019 12:00
Hey guys so i need to know what is _nh3+> nh4oh ~chemistry~
Answers: 1

Chemistry, 22.06.2019 12:20
Which is an example of the practical pursuit of alchemy? a. forming perfect substances. b. transforming base metals. c. developing metalworking techniques. d. linking spiritual characteristics with material substances.
Answers: 1

Chemistry, 22.06.2019 17:30
What type of organic molecule comprises the majority of a potato?
Answers: 1
You know the right answer?
A seed of CuSO4.5H20 with a mass of 0.500 g was carefully placed into a saturated solution of copper...
Questions














Mathematics, 07.09.2019 03:20

Mathematics, 07.09.2019 03:20

English, 07.09.2019 03:20




 :42 3.128 39.823 686
:42 3.128 39.823 686