

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:10
A+b→2c when the reaction begins, the researcher records that the rate of reaction is such that 1 mole of a is consumed per minute. after making changes to the reaction, the researcher notes that 2 moles of a are consumed per minute. what change could the researcher have made to effect this change?
Answers: 1


Chemistry, 23.06.2019 03:30
Why do electrons further from the nucleus have more energy
Answers: 1

Chemistry, 23.06.2019 03:30
The molar mass of iron(fe) is 55.8 g/mol. what is the mass in grams of 2.25 moles of iron?
Answers: 1
You know the right answer?
The value of Ka for phenol (a weak acid), C6H5OH, is 1.00×10-10. Write the equation for the reaction...
Questions











Social Studies, 30.12.2019 23:31




Computers and Technology, 30.12.2019 23:31





![Ka=\frac{[C_6H_5O^-][H^+]}{[C_6H_5OH]}](/tpl/images/0706/1269/e7022.png)
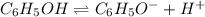
![K=Ka=\frac{[C_6H_5O^-][H^+]}{[C_6H_5OH]}](/tpl/images/0706/1269/c3ab7.png)


