
Chemistry, 14.07.2020 01:01 shyanne9364
A student takes a sample of KOH solution and dilutes it to 100.00 mL of water. The student determines that the diluted solution is 0.15 M KOH, but has forgotten to record the volume of the original sample. The concentration of the original solution is 3.817 M. What was the volume of the original sample (in mL)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Consider the point on the plot where 10.0 g of naoh have been added. what amount of naoh, in moles, has been added? 0.308 mol fecl3 initially present
Answers: 1

Chemistry, 21.06.2019 21:00
What pressure will be exerted by 0.675 moles of a gas at 25*c if it is in a 0.750-l container?
Answers: 1

Chemistry, 21.06.2019 22:30
Naoki's bicycle has a mass of 10 kg. if naoki sits on her bicycle and starts pedaling with a force of 168 n, causing an acceleration of 2.8 m/s2, what is naoki's mass?
Answers: 1

Chemistry, 21.06.2019 23:00
What is the maximum amount of al2(so4)3 which could be formed from 15.84 g of al and 12.89 g of cuso4?
Answers: 2
You know the right answer?
A student takes a sample of KOH solution and dilutes it to 100.00 mL of water. The student determine...
Questions

English, 18.11.2020 22:40


English, 18.11.2020 22:40



Medicine, 18.11.2020 22:40





Mathematics, 18.11.2020 22:40

English, 18.11.2020 22:40



Mathematics, 18.11.2020 22:40

Social Studies, 18.11.2020 22:40


Mathematics, 18.11.2020 22:40

Arts, 18.11.2020 22:40

Mathematics, 18.11.2020 22:40

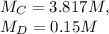
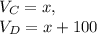
 " solve for x, the volume of the original sample,
" solve for x, the volume of the original sample,

