
Chemistry, 14.07.2020 01:01 laurachealsy923
Calculate Ecell at 80 ºC for a voltaic cell based on the following redox reaction: H2(g, 1.25 atm) + 2AgCl(s) → 2Ag(s) + 2H+(aq, 0.10 M) + 2Cl–(aq, 1.5 M) The standard cell potential Eºcell = +0.18 V at this temperature.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:30
Two atoms interact with each other as shown by the equation. complete the equation by filling in the missing parts. 1 2 3 4 5 h he li
Answers: 2

Chemistry, 22.06.2019 07:30
Which of the following best supports the concept that genetic information is passed on to offspring from both of their parents, not just one?
Answers: 2

Chemistry, 22.06.2019 09:00
The diagram below shows a cell placed in a solution.a cell is shown placed inside a beaker. it is labeled cell. the solution inside the beaker is labeled 40% salt solution and the solution inside the cell is labeled 20% salt solution.only water is allowed to move in and out of the cell. what will most likely happen to the cell? it will expand as water moves out of it. it will shrink as water moves out of it.it will expand as water moves into it. it will shrink as water moves into it.
Answers: 2

You know the right answer?
Calculate Ecell at 80 ºC for a voltaic cell based on the following redox reaction: H2(g, 1.25 atm) +...
Questions


Mathematics, 18.08.2020 15:01

History, 18.08.2020 15:01


Mathematics, 18.08.2020 15:01

Physics, 18.08.2020 15:01


Social Studies, 18.08.2020 15:01

Mathematics, 18.08.2020 15:01




English, 18.08.2020 15:01

History, 18.08.2020 15:01

Mathematics, 18.08.2020 15:01

English, 18.08.2020 15:01

Mathematics, 18.08.2020 15:01



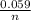 logQ
logQ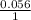 log 0.067
log 0.067

