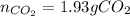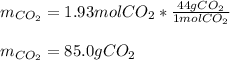Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium
Answers: 1

Chemistry, 22.06.2019 21:00
Which of the following is a physical property flammability heat of combustion solubility and toxicity
Answers: 1


Chemistry, 22.06.2019 22:10
Which aqueous solution of ki freezes at the lowest temperature? 1) 1 mol of ki in 500. g of water 2) 2 mol of ki in 500. g of water 3) 1 mol of ki in 1000. g of water 4) 2 mol of ki in 1000. g of water
Answers: 3
You know the right answer?
Consider the combustion reaction for octane (C8H18), which is a primary component of gasoline.
2C8...
Questions

Social Studies, 14.11.2020 01:00


History, 14.11.2020 01:00

Computers and Technology, 14.11.2020 01:00

Social Studies, 14.11.2020 01:00


Mathematics, 14.11.2020 01:00


Mathematics, 14.11.2020 01:00




Mathematics, 14.11.2020 01:00

Mathematics, 14.11.2020 01:00

Physics, 14.11.2020 01:00

Mathematics, 14.11.2020 01:00


History, 14.11.2020 01:00

Mathematics, 14.11.2020 01:00

Chemistry, 14.11.2020 01:00