
Chemistry, 15.07.2020 01:01 andersonrocksc
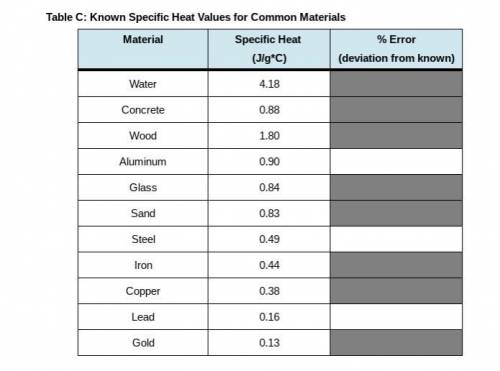
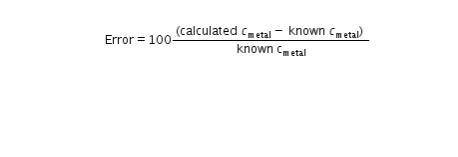
PLEASE I NEED HELP WITH THE BOXES ASAP !! in this last step, return to Step 10 in your Lab Guide to calculate the error between your calculated specific heat of each metal and the known values in Table C. Follow the directions given in your Lab Guide, using this formula: Error = 100 times StartFraction calculated c subscript metal minus known c subscript metal over known C subscript metal EndFraction.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 11:30
Which statement best describes the flow of energy in this scenario
Answers: 1


Chemistry, 22.06.2019 14:30
How can carbon move from "land" to bodies of water? describe the way human impact has lead to increased levels of co2 in the atmosphere.
Answers: 2
You know the right answer?
PLEASE I NEED HELP WITH THE BOXES ASAP !! in this last step, return to Step 10 in your Lab Guide to...
Questions




Business, 26.04.2020 08:37


Mathematics, 26.04.2020 08:37

Mathematics, 26.04.2020 08:37


Geography, 26.04.2020 08:37

History, 26.04.2020 08:37



Biology, 26.04.2020 08:38

Mathematics, 26.04.2020 08:38


Mathematics, 26.04.2020 08:38

Mathematics, 26.04.2020 08:38


Mathematics, 26.04.2020 08:38



