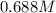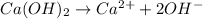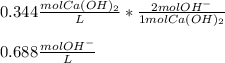Chemistry, 15.07.2020 01:01 kendrawalraven
Determine the [OH⁻] concentration in a 0.344 M Ca(OH)₂ solution.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:50
Select the correct answer how does the heat content of the reaction change in the process of photosynthesis when a glucose molecule is formed? ca the value of is negative the value of qis positive the value of a remains constant the value of a decreases the value of equals zero e
Answers: 2

Chemistry, 22.06.2019 12:10
|using the periodic tablewarm-upuse the periodic table in the tools bar to answer the following questions.what elemental classification does oxygen belongto? done
Answers: 3

Chemistry, 22.06.2019 16:00
Uranium can supply energy for the worlds electricity without admitting harmful greenhouse gases which of these statements best describes an outcome of uranium mining
Answers: 1

Chemistry, 22.06.2019 20:20
Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. in the first step, nitrogen and hydrogen react to form ammonia: (g) (g) (g) in the second step, ammonia and oxygen react to form nitric acid and water: (g) (g) (g) (g) calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 3
You know the right answer?
Determine the [OH⁻] concentration in a 0.344 M Ca(OH)₂ solution....
Questions


Mathematics, 20.11.2019 23:31


Physics, 20.11.2019 23:31

Health, 20.11.2019 23:31

Mathematics, 20.11.2019 23:31

History, 20.11.2019 23:31

Mathematics, 20.11.2019 23:31

Mathematics, 20.11.2019 23:31

History, 20.11.2019 23:31

Mathematics, 20.11.2019 23:31


Mathematics, 20.11.2019 23:31


Mathematics, 20.11.2019 23:31

Mathematics, 20.11.2019 23:31

Physics, 20.11.2019 23:31

History, 20.11.2019 23:31


Chemistry, 20.11.2019 23:31