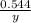Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:30
Which answer lists the fundamental forces in order from strongest to weakest
Answers: 1

Chemistry, 22.06.2019 21:30
How many liters of 3.0 m naoh solution will react with 0.60 liters of 4.0 m h2so4? h2so4 + naoh → na2so4 + h2o 1.2 l 1.6 l 2.4 l 2.8 l
Answers: 3

Chemistry, 22.06.2019 22:30
What is the work done by the electric force to move a 1 c charge from a to b?
Answers: 2

Chemistry, 23.06.2019 00:30
How can you write e method for the experiment of separating sand from water by filtration process? 1-materials 2-steps 3-conclusion also the same for the separating process of water and salt by filtration or distillation. quick because i need to finish my hw
Answers: 2
You know the right answer?
Small quantities of oxygen can be prepared in the laboratory by heating potassium chlorate, KClO3(s)...
Questions

Arts, 07.10.2019 08:02



Mathematics, 07.10.2019 08:02

World Languages, 07.10.2019 08:02

Mathematics, 07.10.2019 08:02

History, 07.10.2019 08:02



Mathematics, 07.10.2019 08:02


Mathematics, 07.10.2019 08:02

Mathematics, 07.10.2019 08:02

History, 07.10.2019 08:02





History, 07.10.2019 08:02

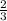 =
=