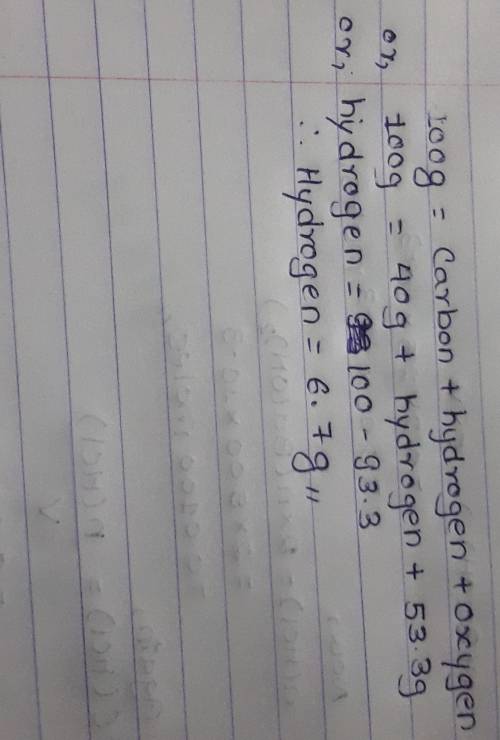Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3

Chemistry, 22.06.2019 13:00
In a copper wire, a temperature increase is the result of which of the following
Answers: 1

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 2

Chemistry, 22.06.2019 17:00
Which property of a rock remains unchanged by mechanical weathering? a. total surface area b. size and shape c. mineral composition d. sharpness
Answers: 1
You know the right answer?
A 100.0 g sample of sugar contains carbon, hydrogen, and oxygen. The sample contains 40.0 g of carbo...
Questions

History, 25.08.2019 06:00

Mathematics, 25.08.2019 06:00


Biology, 25.08.2019 06:00

Biology, 25.08.2019 06:00


Mathematics, 25.08.2019 06:00

Spanish, 25.08.2019 06:00

Computers and Technology, 25.08.2019 06:00


Mathematics, 25.08.2019 06:00


Chemistry, 25.08.2019 06:00

Geography, 25.08.2019 06:00

Biology, 25.08.2019 06:00

Chemistry, 25.08.2019 06:00




History, 25.08.2019 06:00