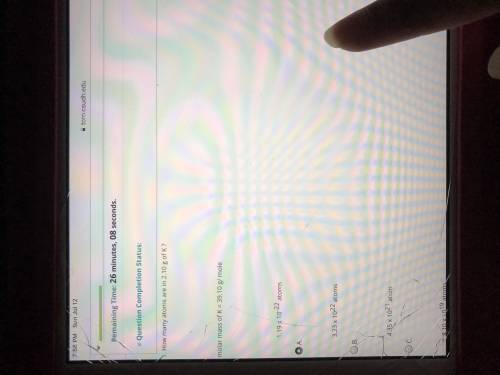
How many atoms are in 2.10 g of K? molar mass of K=39.10g/mole.
...


Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
The image shows a process that releases nuclear energy which statement best identifies the process shown the process must be fusion because energy is released the process must be fusion because of have your nucleus formed a smaller nuclei the process must be fission because a large nucleus breaks into smaller nuclei the process must be fission because neutrons are formed
Answers: 1

Chemistry, 22.06.2019 17:10
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2


Chemistry, 23.06.2019 01:00
The primary products of complete combustion of fossil fuels are a. carbon dioxide and water b. methane and water c. carbon monoxide and water d. carbon dioxide and carbon monoxide
Answers: 1
You know the right answer?
Questions

Computers and Technology, 04.01.2021 19:10

Mathematics, 04.01.2021 19:10




Mathematics, 04.01.2021 19:10


Mathematics, 04.01.2021 19:10


Mathematics, 04.01.2021 19:10

English, 04.01.2021 19:10

Mathematics, 04.01.2021 19:10


Mathematics, 04.01.2021 19:10


History, 04.01.2021 19:10


Chemistry, 04.01.2021 19:10

Mathematics, 04.01.2021 19:10

Biology, 04.01.2021 19:10