Chemistry, 14.07.2020 01:01 gissellesolorza
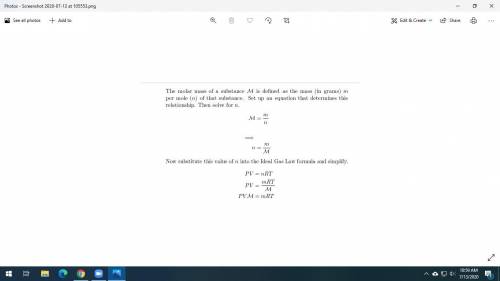
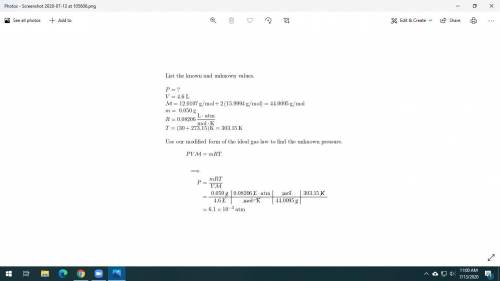
Dry ice is solid carbon dioxide. A 0.050-g sample of dry ice is placed in an evacuated 4.6-L vessel at 30 °C. Calculate the pressure inside the vessel after all the dry ice has been converted to CO2 gas.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:20
Match the acid base pairs by arranging the acid name with the conjugate base formula. hydrogen carbonate hydrogen phosphate carbonic acid read water sulfuric acid phosphoric acid a. co32- b. hso4- c. hco3- d. po43- e. h2po4- f. oh-
Answers: 1

Chemistry, 22.06.2019 04:00
14. many depressants reduce small muscle control, making it harder for a. you to steer b. your mind to consider complex problems c. the eye to scan, focus, or stay still d. the kidneys to filter alcohol out of the bloodstream
Answers: 3

Chemistry, 22.06.2019 05:30
Describe the interaction that occurs between two objects with the same electrical charge.
Answers: 1

Chemistry, 22.06.2019 05:50
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1
You know the right answer?
Dry ice is solid carbon dioxide. A 0.050-g sample of dry ice is placed in an evacuated 4.6-L vessel...
Questions



Mathematics, 03.06.2020 13:57


Mathematics, 03.06.2020 13:57

Mathematics, 03.06.2020 13:57


Mathematics, 03.06.2020 13:57

Physics, 03.06.2020 13:57

Health, 03.06.2020 13:57



Mathematics, 03.06.2020 13:57

English, 03.06.2020 13:57

Mathematics, 03.06.2020 13:57


Social Studies, 03.06.2020 13:57