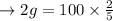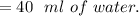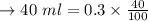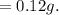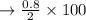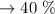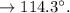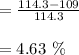Chemistry, 15.07.2020 02:01 enriquerer12
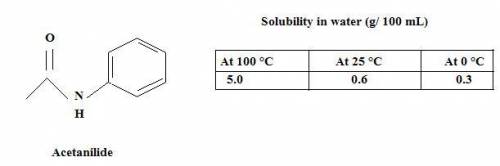
You need to purify 2.0 grams of an impure sample of Acetanilide. The sample is contaminated with aniline. After the purification is complete you isolate 0.8 grams of acetanilide and record a melting point range of 108-110 °C. Complete the following calculations and show your work.
a. Calculate the minimum amount of distilled water you would use to complete the recrystallization.
b. How much acetanilide will still be dissolved in solution even after the sample is cooled to 0 °C?
c. Calculate the % recovery and the % error for the melting point.
d. Why is the percent recovery less than 100%? Describe multiple sources for loss of sample.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 12:20
Achemistry student weighs out 0.306 g of citric acid (h3c6h5o7), a triprotic acid, into a 250 ml volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with 0.1000 m naoh solution. calculate the volume of naoh solution the student will need to add to reach the final equivalence point. be sure your answer has the correct number of significant digits.
Answers: 3

Chemistry, 23.06.2019 01:00
Which is true concerning the products and reactants of photosynthesis and cellular respiration? a. the products of photosynthesis are sugars and the reactants of cellular respiration are starches. b. the products of photosynthesis are reactants in cellular respiration. c. oxygen is needed for photosynthesis and is given off in cellular respiration.
Answers: 2

You know the right answer?
You need to purify 2.0 grams of an impure sample of Acetanilide. The sample is contaminated with ani...
Questions

English, 03.01.2020 22:31




Computers and Technology, 03.01.2020 22:31


Social Studies, 03.01.2020 22:31

Social Studies, 03.01.2020 22:31







Social Studies, 03.01.2020 22:31

Business, 03.01.2020 22:31


English, 03.01.2020 22:31