Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Substance x has a fixed volume, and the attraction between its particles is strong .substance y had widely spread out particles and can be compressed what can most likely be concluded about these substances
Answers: 2

Chemistry, 22.06.2019 04:30
Which is a chemical property of iron? a. it forms iron oxide (rust) when exposed to moisture and air. b. it is a gray–black metal that is hard to the touch. c. it has a melting point of 2795°f (1536°c). d. it is a good conductor of heat
Answers: 2

Chemistry, 22.06.2019 05:40
Calculate: select the worksheet tab. this tab you calculate the analyte concentration. fill in the first set of boxes ("moles h2so4" and "moles naoh") based on the coefficients in the balanced equation. (if there is no coefficient, the value is 1.) record the appropriate volumes in the "ml naoh" and "ml h2so4" boxes. record the concentration of the titrant in the m naoh box. click calculate. what is the concentration listed
Answers: 2

Chemistry, 22.06.2019 09:20
Sugar is dissolved in water. which is the solute? sugar neither both water
Answers: 1
You know the right answer?
Calculate the mass of magnesium carbonate ( MgCO3), in grams, required to produce 110.0 g of carbon...
Questions


Mathematics, 03.11.2020 05:30

Mathematics, 03.11.2020 05:30

Mathematics, 03.11.2020 05:30



Chemistry, 03.11.2020 05:30

Mathematics, 03.11.2020 05:30

English, 03.11.2020 05:30

History, 03.11.2020 05:30

Mathematics, 03.11.2020 05:30

Mathematics, 03.11.2020 05:30

Advanced Placement (AP), 03.11.2020 05:30


Mathematics, 03.11.2020 05:30

Mathematics, 03.11.2020 05:30



Mathematics, 03.11.2020 05:30

English, 03.11.2020 05:30

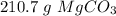
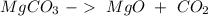
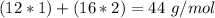
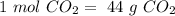 . With this in mind, we can calculate the moles:
. With this in mind, we can calculate the moles: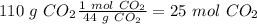
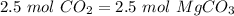
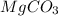 (
(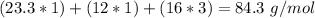 . With this in mind, we can calculate the grams of magnesium carbonate:
. With this in mind, we can calculate the grams of magnesium carbonate: