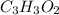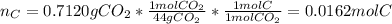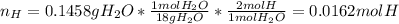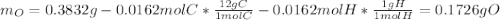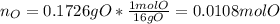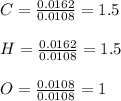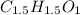Chemistry, 15.07.2020 03:01 shoreelinee1337
A 0.3832-g sample of a compound known to contain only carbon, hydrogen, and oxygen was burned in oxygen to yield 0.7120 g of CO2 and 0.1458 g of H2O. What is the empirical formula of the compound

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:00
How many liters of water vapor can be produced if 108 grams of methane gas (ch4) are combusted at 312 k and 0.98 atm? show all work. pls ! will mark as brainliest
Answers: 1


Chemistry, 22.06.2019 20:00
Nitrogen dioxide decomposes according to the reaction 2 no2(g) ⇌ 2 no(g) + o2(g) where kp = 4.48 × 10−13 at a certain temperature. if 0.70 atm of no2 is added to a container and allowed to come to equilibrium, what are the equilibrium partial pressures of no(g) and o2(g)
Answers: 2

You know the right answer?
A 0.3832-g sample of a compound known to contain only carbon, hydrogen, and oxygen was burned in oxy...
Questions

English, 08.03.2021 22:50




Chemistry, 08.03.2021 22:50



Mathematics, 08.03.2021 22:50



Computers and Technology, 08.03.2021 23:00

Mathematics, 08.03.2021 23:00

Social Studies, 08.03.2021 23:00

English, 08.03.2021 23:00

History, 08.03.2021 23:00


English, 08.03.2021 23:00