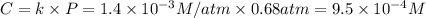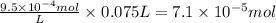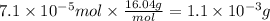Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Explain how the integumentary system plays a crucial role in the ability to maintain homeoestasis
Answers: 1

Chemistry, 22.06.2019 04:00
Write the balanced equation for a reaction between aqueous nitric acid (hno3) and solid lithium metal (this is a single replacement reaction)
Answers: 1

Chemistry, 22.06.2019 06:40
Ted and emily played a mixed doubles tennis match against jack and brenda. in the second match. ted and brenda played against jack and emily. which type of chemical reaction does the situation demonstrate?
Answers: 3

Chemistry, 22.06.2019 07:50
Which of the following electromagnetic waves can create ions?
Answers: 2
You know the right answer?
At 25.0°C the Henry's Law constant for methane CH4 gas in water is ×1.410−3/Matm.
Calculate the mas...
Questions

English, 13.02.2021 17:20

Mathematics, 13.02.2021 17:20

Mathematics, 13.02.2021 17:20



English, 13.02.2021 17:20

Mathematics, 13.02.2021 17:20



Mathematics, 13.02.2021 17:20




Chemistry, 13.02.2021 17:30


Biology, 13.02.2021 17:30



English, 13.02.2021 17:30

Social Studies, 13.02.2021 17:30