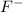Chemistry, 15.07.2020 04:01 Lydiaxqueen
When 100.0 mL of 0.40 M of HF and 100.0 mL of 0.40 M of NaOH are mixed, the resulting mixture is:a. a strong acid b. a strong base c. a weak acid d. a weak base e. a buffer

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:30
What volume of a 2.00 m stock solution of naoh is needed to prepare 150. ml of 0.40 m solution?
Answers: 2

Chemistry, 22.06.2019 04:30
How much energy is made when a pice of wood burns. how do you know
Answers: 2

Chemistry, 22.06.2019 11:30
Voltaic cells produce a positive overall charge. what does this indicate? a. the reaction is likely to be endothermic. b. the reaction is spontaneous. c. the reaction is not likely to occur. d. the reaction is not spontaneous.
Answers: 3

Chemistry, 22.06.2019 13:30
Which is true of a liquid? it has a definite volume but not a definite mass.it has a definite mass but not a definite volume.it has a definite volume but not a definite shape.it has a definite shape but not a definite volume.
Answers: 2
You know the right answer?
When 100.0 mL of 0.40 M of HF and 100.0 mL of 0.40 M of NaOH are mixed, the resulting mixture is:a....
Questions


Social Studies, 11.02.2020 18:28




Computers and Technology, 11.02.2020 18:28

Computers and Technology, 11.02.2020 18:28





Mathematics, 11.02.2020 18:28




Mathematics, 11.02.2020 18:28

Mathematics, 11.02.2020 18:28


Mathematics, 11.02.2020 18:28

English, 11.02.2020 18:28

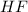 and
and 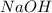 . So:
. So: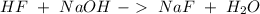
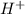
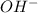
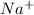 and the anion is
and the anion is