
Chemistry, 15.07.2020 05:01 davidb1113
What molar ratio of sodium acetate to acetic acid should be used to prepare a buffer with pH = 4.5? Ka acetic acid = 1.8 x 10-5.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Analyze how limestone is weathered and identify the features that are formed as a result of this dissolution
Answers: 1


Chemistry, 22.06.2019 16:10
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.300 m , [b] = 1.05 m , and [c] = 0.550 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.140 m and [c] = 0.710 m . calculate the value of the equilibrium constant, kc.
Answers: 1

You know the right answer?
What molar ratio of sodium acetate to acetic acid should be used to prepare a buffer with pH = 4.5?...
Questions

History, 24.08.2019 10:00


Mathematics, 24.08.2019 10:00

Mathematics, 24.08.2019 10:00





Mathematics, 24.08.2019 10:00

History, 24.08.2019 10:00

Mathematics, 24.08.2019 10:00

Mathematics, 24.08.2019 10:00

History, 24.08.2019 10:00

Mathematics, 24.08.2019 10:00



English, 24.08.2019 10:00

Social Studies, 24.08.2019 10:00


Mathematics, 24.08.2019 10:00

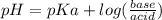 . Since we were given Ka and pH, we can find the ratio.
. Since we were given Ka and pH, we can find the ratio.

