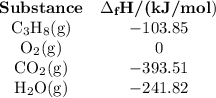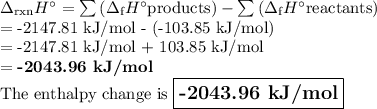Chemistry, 15.07.2020 20:01 mariakelley15
Step 3: Determine the amount of energy change in the reaction. a) Use the table of enthalpy values (Table A) provided in the Student Worksheet to locate the enthalpy of formation (∆Hf) for each reactant and each product. Record these values along with the reactants and products in Table B of the Student Worksheet. b) Determine the total enthalpy of the reactants and the total enthalpy of the products. Record these values in Table C of the Student Worksheet. c) Use the following formula to find the net change in enthalpy for the reaction and to determine whether the reaction is endothermic or exothermic.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:30
Which answer lists the fundamental forces in order from strongest to weakest
Answers: 1

Chemistry, 22.06.2019 05:30
Choose all the answers that apply. as ocean depth increases, temperature decreases temperature increases pressure increases pressure decreases salinity increases density increases
Answers: 2

Chemistry, 22.06.2019 09:10
Which class of molecules functions as chemical signals? hormones water carbohydrates proteins
Answers: 1

Chemistry, 23.06.2019 08:00
If the solubility of a gas in water is 1.22 g/l at 2.75 atm, what is its solubility (in g/l) at 1.0 atm?
Answers: 1
You know the right answer?
Step 3: Determine the amount of energy change in the reaction. a) Use the table of enthalpy values (...
Questions


Mathematics, 31.01.2020 10:47


Computers and Technology, 31.01.2020 10:47


English, 31.01.2020 10:47

History, 31.01.2020 10:47


Biology, 31.01.2020 10:47

Mathematics, 31.01.2020 10:47

History, 31.01.2020 10:47

Mathematics, 31.01.2020 10:47


Mathematics, 31.01.2020 10:47


Mathematics, 31.01.2020 10:47

Chemistry, 31.01.2020 10:47


English, 31.01.2020 10:47

Spanish, 31.01.2020 10:47