
Chemistry, 15.07.2020 21:01 duncanswart1558
A sample of Ne gas has a pressure of 654 mmHg with an unknown volume. The gas has a pressure of 345 mmHg when the volume is 495mL with no change in temperature or amount of gas. What is the initial volume in milliliters of the gas?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Joseph has hypothesized that sound travels in waves. if he were following the scientific method, what should he do next? a. ask a question. b. test the hypothesis. c. study the results. d. tell other scientists about his hypothesis.
Answers: 1

Chemistry, 22.06.2019 17:30
A650 ml sodium bromine solution has a bromide ion concentration of 0.245 m. what is the mass (g) of sodium bromide in solution? a) 103.b)0.00155.c)16400.d) 16.4.e) 0.159
Answers: 2

Chemistry, 22.06.2019 20:20
Which formula equation represents the burning of sulfur to produce sulfur dioxide? s(s) + o2(g) 4502(9) 2h2s(s) + 302(g) —> 2h20(0) + 2502(9) 4fes2+1102 —> 2fe2o3 + 8502 2802(g) + o2(9) v205 , 2503(9)
Answers: 1

Chemistry, 22.06.2019 21:30
Achemical reaction is done in the setup shown, resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 1
You know the right answer?
A sample of Ne gas has a pressure of 654 mmHg with an unknown volume. The gas has a pressure of 345...
Questions

Mathematics, 06.04.2021 07:40

Mathematics, 06.04.2021 07:40

Mathematics, 06.04.2021 07:40


Mathematics, 06.04.2021 07:40


Mathematics, 06.04.2021 07:40



Mathematics, 06.04.2021 07:40


Chemistry, 06.04.2021 07:40

Mathematics, 06.04.2021 07:40



English, 06.04.2021 07:40


English, 06.04.2021 07:40

Arts, 06.04.2021 07:40

Mathematics, 06.04.2021 07:40

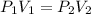
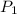 represent the initial pressure
represent the initial pressure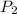 represent the final pressure
represent the final pressure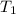 represent the initial temperature
represent the initial temperature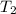 represent the final temperature
represent the final temperature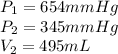


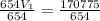
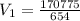
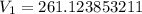
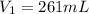 (Approximated)
(Approximated)

