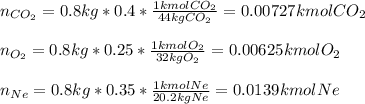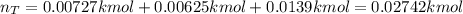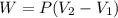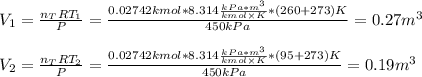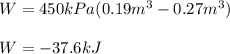A closed, frictionless piston-cylinder contains a gas mixture with the following composition on a mass basis: 40% carbon dioxide, 25% oxygen, 35% neon The cylinder contains 0.8 kg of the mixture at 260 oC and 450 kPa. Determine the magnitude and direction of work when the mixture undergoes an isobaric process to 95 oC.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:50
8) a mixture of he, ne and ar has a pressure of 7.85 atm. if the ne has a mole fraction of 0.47 and 8) ar has a mole fraction of 0.23, what is the pressure of he? a) 4.2 atm b) 3.7 atm c) 5.5 atm d) 2.4 atm e) 1.8 atm
Answers: 1

Chemistry, 22.06.2019 11:40
Which of these expressions are correct variations of the combined gas law? p1v1t2 = p2v2t1 both
Answers: 2


Chemistry, 23.06.2019 10:30
When a chemist collects hydrogen gas over water, she ends up with a mixture of hydrogen and water vapor in her collecting bottle. if the pressure in the collecting bottle is 97.1 kilopascals and the vapor pressure of the water is 3.2 kilopascals, what is the partial pressure of the hydrogen?
Answers: 1
You know the right answer?
A closed, frictionless piston-cylinder contains a gas mixture with the following composition on a ma...
Questions




Mathematics, 05.11.2020 01:40


Mathematics, 05.11.2020 01:40

Computers and Technology, 05.11.2020 01:40


Mathematics, 05.11.2020 01:40

Mathematics, 05.11.2020 01:40



Mathematics, 05.11.2020 01:40




English, 05.11.2020 01:40

Mathematics, 05.11.2020 01:40

Law, 05.11.2020 01:40