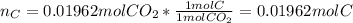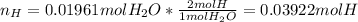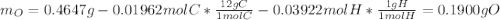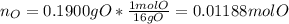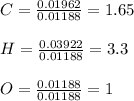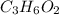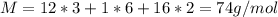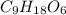Chemistry, 15.07.2020 22:01 emilymendes546
A 0.4647-g sample of a compound known to contain only carbon, hydrogen, and oxygen was burned in oxygen to yield 0.01962 mol of CO2 and 0.01961 mol of H2O. The empirical formula of the compound was found to be C3H6O2. Show how this was calculated. What does the empirical formula tell you about the compound? The molar mass of the actual compound was found to be 222.27g/mol. Find the molecular formula of this compound. What does the molecular formula tell you about the compound? Can you see what type of functional group this compound could have?

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 06:00
What type of electromagnetic radiation has a shorter wavelength than blue light
Answers: 2

Chemistry, 22.06.2019 13:30
How many protons, electrons, and neutrons are in each of the following isotopes? a. zirconium-90 b. palladium-108 c. bromine-81 d. antimony-123
Answers: 1

Chemistry, 22.06.2019 20:00
What happens to the temperature of a substance when the average kinetic energy of its particles increases?
Answers: 3
You know the right answer?
A 0.4647-g sample of a compound known to contain only carbon, hydrogen, and oxygen was burned in oxy...
Questions


Physics, 14.12.2019 04:31

English, 14.12.2019 04:31

Mathematics, 14.12.2019 04:31


Biology, 14.12.2019 04:31

Computers and Technology, 14.12.2019 04:31


History, 14.12.2019 04:31

Spanish, 14.12.2019 04:31



Mathematics, 14.12.2019 04:31

English, 14.12.2019 04:31