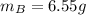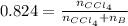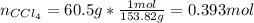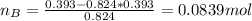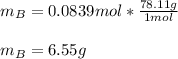Chemistry, 16.07.2020 01:01 jennamcasey94
A solution was made by mixing benzene () and carbon tetrachloride (). Given that the mole fraction of carbon tetrachloride is 0.824 in the solution obtained from 60.5 g , calculate the mass of benzene used. Mass

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:40
What kind of ion is contained in salts that produce an acidic solution? a positive ion that attracts a proton from water a positive ion that releases a proton to water a negative ion that attracts a proton from water a negative ion that releases a proton to water
Answers: 1

Chemistry, 22.06.2019 03:00
Flourine is found to undergo 10% radioactivity decay in 366 minutes determine its halflife
Answers: 3

Chemistry, 22.06.2019 07:10
Remember to use the proper number of significant figures and leading zeros in all calculations.gelatin has a density of 1.27 g/cm³. if you have a blob of gelatin dessert that fills a 2.0 liter bottle, what is its mass? 2540 g2500 g3.9 x 10-43.937x 10-4
Answers: 3

Chemistry, 22.06.2019 07:30
In a reaction (at equilibrium) that makes more moles of gas than it consumes, what is the effect of increasing the pressure?
Answers: 1
You know the right answer?
A solution was made by mixing benzene () and carbon tetrachloride (). Given that the mole fraction o...
Questions


Mathematics, 04.11.2020 21:40

Geography, 04.11.2020 21:40


Mathematics, 04.11.2020 21:40


Social Studies, 04.11.2020 21:40

English, 04.11.2020 21:40




Mathematics, 04.11.2020 21:40

Biology, 04.11.2020 21:40



English, 04.11.2020 21:40


Social Studies, 04.11.2020 21:40


English, 04.11.2020 21:40