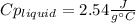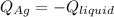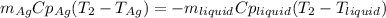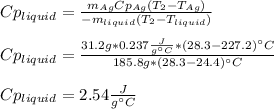Chemistry, 16.07.2020 01:01 makaylaf9479
3. A 31.2-g piece of silver (s = 0.237 J/(g · °C)), initially at 277.2°C, is added to 185.8 g of a liquid, initially at 24.4°C, in an insulated container. The final temperature of the metal–liquid mixture at equilibrium is 28.3°C. What is the specific heat of the liquid? Neglect the heat capacity of the container.

Answers: 1


Another question on Chemistry



Chemistry, 23.06.2019 06:10
2. what two items do autotrophs take from the environment to produce their food? 3. what are the two items that are released during transpiration from leaves? 4. what are the two membranes of the system? a.what are the two stages of photosynthesis? what are the two parts of photosynthesis?
Answers: 2

You know the right answer?
3. A 31.2-g piece of silver (s = 0.237 J/(g · °C)), initially at 277.2°C, is added to 185.8 g of a l...
Questions



Social Studies, 10.11.2020 20:00



Biology, 10.11.2020 20:00



Chemistry, 10.11.2020 20:00

Mathematics, 10.11.2020 20:00

Mathematics, 10.11.2020 20:00

Business, 10.11.2020 20:00


Mathematics, 10.11.2020 20:00



Social Studies, 10.11.2020 20:00


History, 10.11.2020 20:00