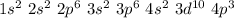Chemistry, 16.07.2020 03:01 genyjoannerubiera
A ground state atom of As could not have any electrons with which of the following configurations? A) n = 3, ℓ = 1, mℓ = 0, ms = +½ B) n = 4, ℓ = 2, mℓ = 0, ms = +½ C) n = 4, ℓ = 0, mℓ = 0, ms = +½ D) n = 3, ℓ = 2, mℓ = 0, ms = +½ E) n = 4, ℓ = 1, mℓ = 0, ms = +½

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:00
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2

Chemistry, 22.06.2019 15:00
Why does a plastic bottle that is sealed at a high altitude change it’s shape when taken to lower altitude
Answers: 2

Chemistry, 22.06.2019 22:50
At the current rate, a graph of carbon dioxide produced by fossil fuels over time would slope upward slope downward be horizontal be vertical
Answers: 3

Chemistry, 23.06.2019 09:20
Four statements about the development of the atomic model are shown below. a: electrons have wavelike properties. b: atoms have small, negatively charged particles. c. the center of an atom is a small, dense nucleus. d: atoms are hard, indivisible spheres. which order of statements represents the historical development of the atomic model? c-d-a-b c-d-b-a d— в-а — с d-b-c-a
Answers: 1
You know the right answer?
A ground state atom of As could not have any electrons with which of the following configurations? A...
Questions


English, 11.12.2019 01:31

Mathematics, 11.12.2019 01:31




Mathematics, 11.12.2019 01:31


Geography, 11.12.2019 01:31



Business, 11.12.2019 01:31

Mathematics, 11.12.2019 01:31




Mathematics, 11.12.2019 01:31

Mathematics, 11.12.2019 01:31


Mathematics, 11.12.2019 01:31