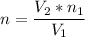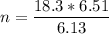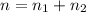Chemistry, 16.07.2020 17:01 silasjob09
A flexible container at an initial volume of 6.13 L contains 6.51 mol of gas. More gas is then added to the container until it reaches a final volume of 18.3 L. Assuming the pressure and temperature of the gas remain constant, calculate the number of moles of gas added to the container.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 18:00
Answer asap need to be answered by wednesday morning explain how a buffer works, using an ethanoic acid / sodium ethanoate system including how the system resists changes in ph upon addition of a small amount of base and upon addition of a small amount of acid respectively. include the following calculations in your i. calculate the ph of a solution made by mixing 25cm3 0.1m ch3cooh and 40cm3 0.1m ch3coo-na+. [ka = 1.74 x 10-5 m] ii. calculate the ph following the addition of a 10cm3 portion of 0.08 m naoh to 500cm3 of this buffer solution. iii. calculate the ph following the addition of a 10cm3 portion of 0.08 m hcl to 200cm3 of the original buffer solution.
Answers: 3

Chemistry, 22.06.2019 18:00
Heat is the total potential energy of a substance that can be transferred. true false
Answers: 1

Chemistry, 22.06.2019 20:10
What would happen to a volleyball left outside in the winter? o o o o a. it would expand. b. it would lose air. c. it would shrink. d. it would explode.
Answers: 2

Chemistry, 23.06.2019 01:20
Use the de broglie's wave equation to find the wavelength of an electron moving at 7.3 × 106 m/s. show your work. note: h = plank's constant (6.62607 x 10-34 j s)
Answers: 1
You know the right answer?
A flexible container at an initial volume of 6.13 L contains 6.51 mol of gas. More gas is then added...
Questions

Mathematics, 13.01.2021 03:50

Social Studies, 13.01.2021 03:50

Mathematics, 13.01.2021 03:50

Mathematics, 13.01.2021 03:50

Mathematics, 13.01.2021 03:50




Mathematics, 13.01.2021 03:50

Mathematics, 13.01.2021 03:50



Health, 13.01.2021 03:50

Mathematics, 13.01.2021 03:50

Mathematics, 13.01.2021 03:50

Physics, 13.01.2021 03:50

Mathematics, 13.01.2021 03:50



Mathematics, 13.01.2021 03:50