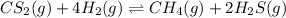The reaction system
CS2(g) + 4H2(g) CH4(g) + 2H2S(g)
is at equilibrium. Which of the followi...

Chemistry, 17.07.2020 19:01 haleynicole351ovewbg
The reaction system
CS2(g) + 4H2(g) CH4(g) + 2H2S(g)
is at equilibrium. Which of the following statements describes the behavior of the system if the partial pressure of hydrogen is doubled?
a. As equilibrium is reestablished, the partial pressure of methane, CH4, decreases.
b. As equilibrium is reestablished, the partial pressure of carbon disulfide increases.
c. As equilibrium is reestablished, all the partial pressures will decrease.
d. As equilibrium is reestablished, the partial pressure of hydrogen sulfide decreases.
e. As equilibrium is reestablished, the partial pressure of hydrogen decreases.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:10
How is 0.00235 expressed in proper scientific notation? a. 2.35 × 10-3 b. 0.235 × 10-2 c. 2.35 d. 2.35 × 103
Answers: 1

Chemistry, 22.06.2019 06:40
Which statement is usually true about the relationship between activation energy and reaction rates? low activation energy barriers result in low rates. high activation energy barriers result in low rates. low activation energy barriers result in no reaction. high activation energy barriers result in no reaction.
Answers: 3

Chemistry, 22.06.2019 11:00
Which statement correctly identifies the scientific question and describes why the question is scientific? question 1 refers to the supernatural.question 2 reflects a moral or social value.question 3 refers to something that can be measured.question 4 reflects a question that can’t be observed.
Answers: 1

Chemistry, 23.06.2019 00:30
When a beta particle is emitted, the mass number of the nucleus a. decreases by one b. increases by one c. remains the same d. decreases by two
Answers: 2
You know the right answer?
Questions


Mathematics, 30.11.2020 20:30

History, 30.11.2020 20:30

Mathematics, 30.11.2020 20:30



Mathematics, 30.11.2020 20:30




Social Studies, 30.11.2020 20:30


History, 30.11.2020 20:30

Mathematics, 30.11.2020 20:30


Spanish, 30.11.2020 20:30


English, 30.11.2020 20:30

History, 30.11.2020 20:30