
Chemistry, 17.07.2020 20:01 rhussein6452
C2 H6 +02 equals CO2 + H2O what mass of water is produced from three moles of C2 H6?
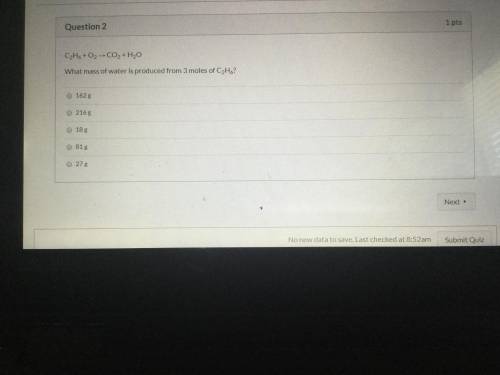

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:40
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1

Chemistry, 22.06.2019 12:30
Acontrol during an experiment. might change remains constant does not exist does change
Answers: 1

Chemistry, 22.06.2019 14:50
Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of electron = 5.486 × 10^-4 amu speed of light = 2.9979 × 10^8 m/s calculate the nuclear binding energy (absolute value) of 3li^6. which has an atomic mass of 6.015126 amu. j/mol.
Answers: 2

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 2
You know the right answer?
C2 H6 +02 equals CO2 + H2O what mass of water is produced from three moles of C2 H6?
...
...
Questions







Physics, 19.01.2021 02:20

Mathematics, 19.01.2021 02:20







Computers and Technology, 19.01.2021 02:20

English, 19.01.2021 02:20






