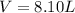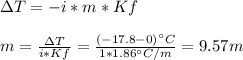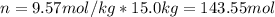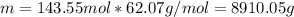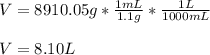Chemistry, 17.07.2020 21:01 stacysadousky
How many liters of ethylene glycol antifreeze (C 2H 6O 2) would you add to your car radiator containing 15.0 L of water if you needed to protect your engine to –17.8°C? (The density of ethylene glycol is 1.1 g/mL. For water, K f = 1.86°C/m.)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:10
Which statement is true about the part of the electromagnetic spectrum that human eyes can detect? it contains only the colors of the rainbow and television waves. o it is divided into seven ranges of wavelengths. it contains ultraviolet, visible, and infrared light. it is divided into nine ranges of wavelengths.
Answers: 2

Chemistry, 22.06.2019 06:00
According to each substances heat of fusion, which of the items below requires more heat to be added per gram of substance to go from solid to liquid? silver sulfur water lead
Answers: 2

Chemistry, 22.06.2019 14:20
7. in the cycle, a virus integrates its dna into the host's dna, and its dna is replicated when the host dna is replicated. a. infectious b. retroviral c. lysogenic d.lytic
Answers: 1

Chemistry, 22.06.2019 15:20
Draw any one of the skeletal structures of a 2° alkyl bromide having the molecular formula of c6h13br and two stereogenic centers. indicate chirality by using wedge and hashed wedge notation. lone pairs do not need to be shown.
Answers: 1
You know the right answer?
How many liters of ethylene glycol antifreeze (C 2H 6O 2) would you add to your car radiator contain...
Questions


Biology, 23.09.2019 09:30

Biology, 23.09.2019 09:30




Mathematics, 23.09.2019 09:30




Mathematics, 23.09.2019 09:30

Biology, 23.09.2019 09:30

Biology, 23.09.2019 09:30

Mathematics, 23.09.2019 09:30

Social Studies, 23.09.2019 09:30



Mathematics, 23.09.2019 09:30

Mathematics, 23.09.2019 09:30