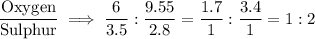Chemistry, 17.07.2020 21:01 maybrieldridge12
Two oxide of sulphur, A and B were analyzed and the results obtained showed that in oxide A,3.50g of sulphur combined with 6.00g of oxygen and in oxide B,2.80g of sulphur combined with 9.55g. Show that this result illustrate the law of multiple proportion

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 13:00
When light strikes a plane mirror, images form in locations where light does not actually reach. it only appears to the observer as though the light were coming from this position. what type of image is formed?
Answers: 2

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and a solid called? identify which way they are transitioning
Answers: 2


Chemistry, 22.06.2019 16:00
As changes in energy levels of electrons increase, the frequencies of atomic line spectra they emit
Answers: 2
You know the right answer?
Two oxide of sulphur, A and B were analyzed and the results obtained showed that in oxide A,3.50g of...
Questions

English, 22.04.2020 23:23

Mathematics, 22.04.2020 23:23


Engineering, 22.04.2020 23:23

Biology, 22.04.2020 23:23

Physics, 22.04.2020 23:23





Mathematics, 22.04.2020 23:24

Biology, 22.04.2020 23:24

Arts, 22.04.2020 23:24

Mathematics, 22.04.2020 23:24

Health, 22.04.2020 23:24



Biology, 22.04.2020 23:24

Mathematics, 22.04.2020 23:24

Mathematics, 22.04.2020 23:24