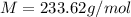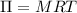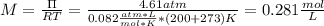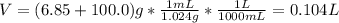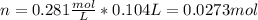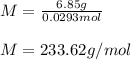Chemistry, 17.07.2020 22:01 SoccerdudeDylan
A solution of 6.85g of a carbohydrate in 100.0g of water has a density of 1.024 g/mL and an osmotic pressure of 4.61 atm at 200C. Calculate molar mass of the carbohydrate

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Summarize possible ways in which phases of matter could combine to form a solution.
Answers: 2

Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 1

Chemistry, 22.06.2019 12:30
Clyde and marilyn are riding a roller coaster. during which section(s) of the track is their potential energy converted to kinetic energy? a. from point b to point c only b. from point b to point d only c. from point a to point b only d. from point a to point b and from point c to point d
Answers: 1

Chemistry, 22.06.2019 13:10
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1
You know the right answer?
A solution of 6.85g of a carbohydrate in 100.0g of water has a density of 1.024 g/mL and an osmotic...
Questions


Physics, 26.04.2021 21:00


Social Studies, 26.04.2021 21:00

History, 26.04.2021 21:00

Mathematics, 26.04.2021 21:00




History, 26.04.2021 21:00

Mathematics, 26.04.2021 21:00





Mathematics, 26.04.2021 21:00

Mathematics, 26.04.2021 21:00