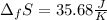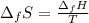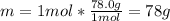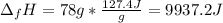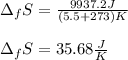Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:40
During trial 2, what allowed you to determine that aluminum was the limiting reactant? check all that apply. all of the copper dissolved. all of the aluminum dissolved. the solution turned clear. the number of grams of copper(ii) chloride used in the reaction was greater than the number of grams of aluminum. the molar ratio of copper(ii) chloride to aluminum was greater than 3: 2, the equation’s molar ratio.
Answers: 2

Chemistry, 22.06.2019 03:00
What happened in 2012 and how does it illustrate the importance of understanding the sun and how it works?
Answers: 3

Chemistry, 22.06.2019 21:00
Once similarity and one difference between a mixture of elements and a mixture of compounds
Answers: 3

Chemistry, 22.06.2019 21:00
What is the chemical formula for the compound formed between sodium and flour one
Answers: 1
You know the right answer?
10. The enthalpy of fusion for benzene (C6H6, 78.0 g/mol) is 127.40 kJ/kg, and its melting point is...
Questions




Physics, 04.07.2019 07:00


Mathematics, 04.07.2019 07:00

Biology, 04.07.2019 07:00

English, 04.07.2019 07:00


English, 04.07.2019 07:00

Biology, 04.07.2019 07:00

Business, 04.07.2019 07:00


English, 04.07.2019 07:00

Biology, 04.07.2019 07:00


Biology, 04.07.2019 07:00

Mathematics, 04.07.2019 07:00

Mathematics, 04.07.2019 07:00

Physics, 04.07.2019 07:00