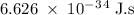Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Select the correct answer. which statement is true about a polarized object? o a. it gains electrons and becomes negatively charged. ob. it gains protons and becomes positively charged. oc. the number of positive and negative charges can be the same. od. it has to be a metal. o e. there is no change in the distribution of the charge in the object. reset next what
Answers: 3

Chemistry, 22.06.2019 02:50
Consider the equilibrium system: 2icl(s) ⇄ i2(s) + cl2(g) which of the following changes will increase the total amount of of cl2 that can be produced? all of the listed answers are correct decreasing the volume of the container removing the cl2 as it is formed adding more icl(s) removing some of the i2(s)
Answers: 1


You know the right answer?
the energy to break 1 mol of C-C bonds is 348 kj/mol. what would be the minimum frequency of a singl...
Questions

Health, 29.09.2020 01:01

Computers and Technology, 29.09.2020 01:01

Mathematics, 29.09.2020 01:01

Mathematics, 29.09.2020 01:01





Chemistry, 29.09.2020 01:01





English, 29.09.2020 01:01

Mathematics, 29.09.2020 01:01

Mathematics, 29.09.2020 01:01


Mathematics, 29.09.2020 01:01


 .
.