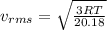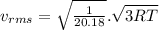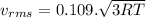Chemistry, 18.07.2020 04:01 Knownothing
A flask contains a mixture of neon Ne, krypton Kr, and radon Rn gases. (Hint: The molar mass of the is Ne 20.180 g/mol, of the Kr is 83.80g/mol, and of the Kr 222g/mol)
(A) Compare the average kinetic energies of the Ne and Kr.
(B) Comparethe average kinetic energies of the Kr and Rn.
(C) Compare the average kinetic energies of the Rn and Ne.
(D) Compare the root-mean-square speeds of the Ne and Kr.
(E) Compare the root-mean-square speeds of the Kr and Rn.
(F) Compare the root-mean-square speeds of the Rn and Ne.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 13:30
What is the molecular formula of a hydrocarbon with m+ = 166? (write the formula with no subscripts, e.g. c4h10.) what is the sum of rings and double bonds in this compound?
Answers: 1

Chemistry, 22.06.2019 17:00
The biosphere of the earth is made up of what compound? organic or inorganic?
Answers: 3

Chemistry, 22.06.2019 20:50
What is the vapor pressure of a solution with a benzene to octane?
Answers: 2

Chemistry, 23.06.2019 01:50
Drag the tiles to the correct locations. each tile can be used more than once, but not all tiles will be used. one or more locations will remain empty. nitrosyl fluoride has the chemical formula nof nitrogen has five valence electrons, oxygen has six, and fluorine has seven. complete the lewis structure for this covalent compound. f n = = = . : : 0 : reset next um. all rights reserved us 2
Answers: 2
You know the right answer?
A flask contains a mixture of neon Ne, krypton Kr, and radon Rn gases. (Hint: The molar mass of the...
Questions


English, 30.01.2020 14:47

Business, 30.01.2020 14:48

Mathematics, 30.01.2020 14:48

History, 30.01.2020 14:48



Chemistry, 30.01.2020 14:48

Social Studies, 30.01.2020 14:48

History, 30.01.2020 14:48


Mathematics, 30.01.2020 14:48



English, 30.01.2020 14:48


Biology, 30.01.2020 14:48



Mathematics, 30.01.2020 14:48