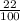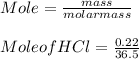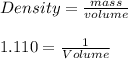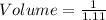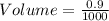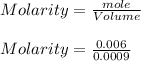Chemistry, 17.07.2020 19:01 GreenHerbz206
The mass percentage of hydrochloric acid within a solution is 22.00%. Given that the density of this solution is 1.110 g/mL, find the molarity of the solution.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 07:00
Which atom or ion is the largest? a. k b. k+ c. ca d. ca2+ e. li
Answers: 1

Chemistry, 22.06.2019 07:30
In a reaction (at equilibrium) that makes more moles of gas than it consumes, what is the effect of increasing the pressure?
Answers: 1

Chemistry, 23.06.2019 13:00
What mass of ca(oh)2 is needed to make 1250ml of a .75m solution?
Answers: 3
You know the right answer?
The mass percentage of hydrochloric acid within a solution is 22.00%. Given that the density of this...
Questions

Computers and Technology, 08.12.2020 01:20

Mathematics, 08.12.2020 01:20

Mathematics, 08.12.2020 01:20

Mathematics, 08.12.2020 01:20


Mathematics, 08.12.2020 01:20

Computers and Technology, 08.12.2020 01:20

Mathematics, 08.12.2020 01:20

Biology, 08.12.2020 01:20

History, 08.12.2020 01:20

Mathematics, 08.12.2020 01:20

Mathematics, 08.12.2020 01:20

Chemistry, 08.12.2020 01:20

Mathematics, 08.12.2020 01:20

Mathematics, 08.12.2020 01:20

English, 08.12.2020 01:20