
Chemistry, 19.07.2020 01:01 josephfoxworth
A solution contains 0.0440 M Ca2 and 0.0940 M Ag. If solid Na3PO4 is added to this mixture, which of the phosphate species would precipitate out of solution first?
A. Na3PO4.
B. Ag3PO4.
C. Ca3(PO4)2
When the second cation just starts to precipitate, what percentage of the first cation remains in solution?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:00
What were the success and failures that came to boyle’s excitements?
Answers: 1

Chemistry, 21.06.2019 22:00
Afamily is one another name for a group on the table of elements.
Answers: 1

Chemistry, 22.06.2019 04:30
Turbo the snail moves across the ground at a pace of 12 feet per day. if the garden is 48 feet away, how many days will it take for the snail to get there?
Answers: 2

Chemistry, 22.06.2019 12:10
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3
You know the right answer?
A solution contains 0.0440 M Ca2 and 0.0940 M Ag. If solid Na3PO4 is added to this mixture, which of...
Questions








History, 08.03.2021 02:40

History, 08.03.2021 02:40




Mathematics, 08.03.2021 02:40

English, 08.03.2021 02:40


History, 08.03.2021 02:40

Social Studies, 08.03.2021 02:40

Biology, 08.03.2021 02:40

Mathematics, 08.03.2021 02:40

Mathematics, 08.03.2021 02:40

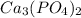 will precipitate out first
will precipitate out first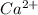 remaining = 12.86%
remaining = 12.86%![[Ca^{2+}] = 0.0440 \ M](/tpl/images/0709/5794/07981.png)
![[Ag^+] = 0.0940 \ M](/tpl/images/0709/5794/e77de.png)
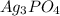
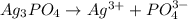
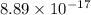
![Ksp = [Ag^+]^3[PO_4^{3-}]](/tpl/images/0709/5794/f170d.png)
![8.89 \times 10 ^{-17} = (0.0940)^3[PO_4^{3-}]](/tpl/images/0709/5794/f1b1a.png)
![\dfrac{8.89 \times 10 ^{-17}}{(0.0940)^3} = [PO_4^{3-}]](/tpl/images/0709/5794/fb0ea.png)
![[PO_4^{3-}] =\dfrac{8.89 \times 10 ^{-17}}{(0.0940)^3}](/tpl/images/0709/5794/36b7f.png)
![[PO_4^{3-}] =1.07 \times 10^{-13}](/tpl/images/0709/5794/d9e0c.png)
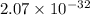
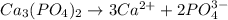
![Ksp = [Ca^{2+}]^3 [PO_4^{3-}]^2](/tpl/images/0709/5794/409cc.png)
![2.07 \times 10^{-33} = (0.0440)^3 [PO_4^{3-}]^2](/tpl/images/0709/5794/a627b.png)
![\dfrac{2.07 \times 10^{-33} }{(0.0440)^3}= [PO_4^{3-}]^2](/tpl/images/0709/5794/4ad8e.png)
![[PO_4^{3-}]^2 = \dfrac{2.07 \times 10^{-33} }{(0.0440)^3}](/tpl/images/0709/5794/fbe62.png)
![[PO_4^{3-}]^2 = 2.43 \times 10^{-29}](/tpl/images/0709/5794/43b90.png)
![[PO_4^{3-}] = \sqrt{2.43 \times 10^{-29}](/tpl/images/0709/5794/0afa0.png)
![[PO_4^{3-}] =4.93 \times 10^{-15}](/tpl/images/0709/5794/19717.png)
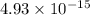 in
in 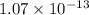
![[Ca^+]](/tpl/images/0709/5794/2b2af.png) when the second cation starts to precipitate ; we have :
when the second cation starts to precipitate ; we have :![2.07 \times 10^{-33} = [Ca^{2+}]^3 (1.07 \times 10^{-13})^2](/tpl/images/0709/5794/8e719.png)
![[Ca^{2+}]^3 = \dfrac{2.07 \times 10^{-33} }{(1.07 \times 10^{-13})^2}](/tpl/images/0709/5794/5de75.png)
![[Ca^{2+}]^3 =1.808 \times 10^{-7}](/tpl/images/0709/5794/9d3e5.png)
![[Ca^{2+}] =\sqrt[3]{1.808 \times 10^{-7}}](/tpl/images/0709/5794/21597.png)
![[Ca^{2+}] =0.00566](/tpl/images/0709/5794/67420.png)
![[Ca^{2+}]](/tpl/images/0709/5794/17576.png) in the solution is 0.00566
in the solution is 0.00566

