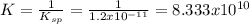Chemistry, 20.07.2020 01:01 littledudefromacross
If 75.0 mL of a 2.63 · 10-3 M NaOH is mixed with 125.0 mL of 1.80 · 10-3 M MgCl2, then calculate the reaction quotient and state if a precipitate will form? The Ksp of the expected precipitate is 1.2 · 10-11.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 2

Chemistry, 22.06.2019 13:00
How many moles of sulfur dioxide are produced when 4.38 moles of oxygen completely react with iron (iv) sulfide
Answers: 2

Chemistry, 22.06.2019 22:00
Ill give u brainliest pls how is mass of carbon conserved during cellular respiration
Answers: 1
You know the right answer?
If 75.0 mL of a 2.63 · 10-3 M NaOH is mixed with 125.0 mL of 1.80 · 10-3 M MgCl2, then calculate the...
Questions


Mathematics, 18.05.2021 16:30




English, 18.05.2021 16:30



Mathematics, 18.05.2021 16:30

Mathematics, 18.05.2021 16:30

Arts, 18.05.2021 16:30


History, 18.05.2021 16:30

Mathematics, 18.05.2021 16:30



Mathematics, 18.05.2021 16:30

Mathematics, 18.05.2021 16:30

Mathematics, 18.05.2021 16:30

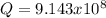
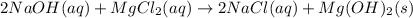
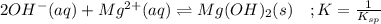
![\frac{1}{K_{sp}}=\frac{1}{[OH^-]^2[Mg^{2+}]}](/tpl/images/0709/8931/c7f27.png)
![[OH^-]=\frac{75.0mL*2.63x10^{-3}M}{75.0mL+125.0mL}=9.86x10^{-4}M](/tpl/images/0709/8931/2f439.png)
![[Mg^{2+}]=\frac{125.0mL*1.80x10^{-3}M}{75.0mL+125.0mL}=1.125x10^{-3}M](/tpl/images/0709/8931/0caa8.png)
![Q=\frac{1}{[OH^-]^2[Mg^{2+}]}=\frac{1}{(9.86x10^{-4})^2*1.125x10^{-3}} =9.143x10^8](/tpl/images/0709/8931/2e242.png)