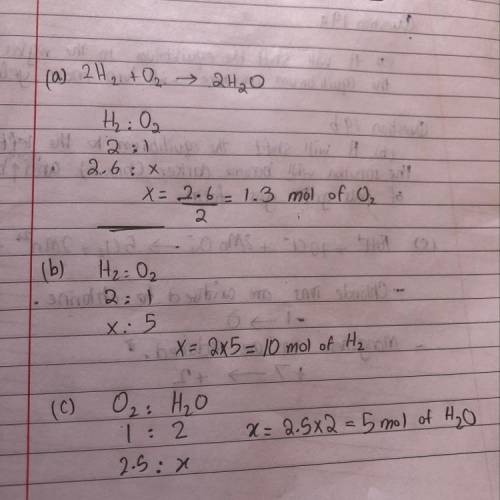The reaction of hydrogen with oxygen produces water.
2H2(g) + O2(8) - 2H2O(g)
a. How many mol...

The reaction of hydrogen with oxygen produces water.
2H2(g) + O2(8) - 2H2O(g)
a. How many moles of O2 are required to react with 2.6 mol
of H2?
b. How many moles of H, are needed to react with 5.0 mol
of O2?
c. How many moles of H2O form when 2.5 mol of O2
reacts?

Answers: 1


Another question on Chemistry



Chemistry, 23.06.2019 01:30
Polar bears give birth and hunt on sea ice. which of the following would polar bears survive during the melting of arctic ice? growing another layer of fur during summer migrate inland to search for different food sources staying put until the ice refreezes sticking to the usual diet of seals
Answers: 1

Chemistry, 23.06.2019 04:40
6) (a) calculate the absorbance of the solution if its concentration is 0.0278 m and its molar extinction coefficient is 35.9 l/(mol cm). the depth of the cell is 5 mm. (b) what is the %t? (7) calculate the absorbance of the solution if the transmitted light intensity is 70% of the initial light beam intensity
Answers: 1
You know the right answer?
Questions

Physics, 20.08.2019 15:00

Social Studies, 20.08.2019 15:00


Mathematics, 20.08.2019 15:00


Chemistry, 20.08.2019 15:00

Mathematics, 20.08.2019 15:00


Chemistry, 20.08.2019 15:00



Mathematics, 20.08.2019 15:00

Mathematics, 20.08.2019 15:00

Mathematics, 20.08.2019 15:00

Geography, 20.08.2019 15:00




Mathematics, 20.08.2019 15:00

English, 20.08.2019 15:00