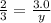Chemistry, 20.07.2020 07:01 memester74
A 3.0 mole sample of KCIO3 was decomposed according to the equation:
2KCIO3(s) ===> 2KCI(s) + 3O2(g)
How many moles of oxygen (O2) will be formed?
o 2.5 moles
4.0 moles
3.0 moles
2.0 moles
4.5 moles

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:50
Which of the following electromagnetic waves can create ions?
Answers: 2

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 2

Chemistry, 23.06.2019 02:20
Which of the following will cause an increase in the acceleration of an object? increase force decrease force increase mass decrease mass
Answers: 1

You know the right answer?
A 3.0 mole sample of KCIO3 was decomposed according to the equation:
2KCIO3(s) ===> 2KCI(s) + 3O...
Questions


Mathematics, 14.10.2019 07:00






Geography, 14.10.2019 07:00


Mathematics, 14.10.2019 07:00


English, 14.10.2019 07:00


History, 14.10.2019 07:00

English, 14.10.2019 07:00



Mathematics, 14.10.2019 07:00


Biology, 14.10.2019 07:00