
In a study of the decomposition of the compound XX via the reaction
X(g)⇌Y(g)+Z(g)X(g)⇌Y(g)+Z(g)
the following concentration-time data were collected:
Time (min)(min) [X](M)[X](M)
0 0.467
1 0.267
2 0.187
3 0.144
4 0.117
5 0.099
6 0.085
7 0.075
Given that the rate constant for the decomposition of hypothetical compound XX is is 1.60 M^−1⋅min^−1. Calculate the concentration of XX after 18.0 minmin .

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Acurium-245 nucleus is hit with a neutron and changes as shown by the equation. complete the equation by filling in the missing parts. 52
Answers: 2


Chemistry, 22.06.2019 16:00
What rule is used to determine how many covalent bonds an element can form? a. the number of covalent bonds is equal to six c the number of covalent bonds is equal to five minus the group number plus the group number b. the number of covalent bonds is equal to eight d. none of the above minus the group number select the best answer from the choices provided
Answers: 2

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium
Answers: 1
You know the right answer?
In a study of the decomposition of the compound XX via the reaction
X(g)⇌Y(g)+Z(g)X(g)⇌Y(g)+Z(g)
Questions

Mathematics, 13.09.2021 19:30

Mathematics, 13.09.2021 19:30



Social Studies, 13.09.2021 19:30


English, 13.09.2021 19:30

Mathematics, 13.09.2021 19:30

Mathematics, 13.09.2021 19:30



Mathematics, 13.09.2021 19:30





Engineering, 13.09.2021 19:40

English, 13.09.2021 19:40


Mathematics, 13.09.2021 19:40

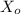 ]"
]"

