
Chemistry, 22.07.2020 06:01 faithtunison
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. An industrial chemist studying this reaction fills a
75.0 L tank with 3.8 mol of sulfur dioxide gas and 7.0 mol of oxygen gas, and when the mixture has come to equilibrium measures the amount of sulfur trioxide
gas to be 1.5 mol
Calculate the concentration equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. Round your answer to 2
significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
The speed of light is around 6.706×10^8 miles per hour. what is the speed of light in units of miles per minute?
Answers: 2

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1

Chemistry, 22.06.2019 15:00
Large helium-filled balloons are used to lift scientific equipment to high altitudes. what is the pressure inside such a balloon if it starts out at sea level with a temperature of 10.0ºc and rises to an altitude where its volume is twenty times the original volume and its temperature is – 50.0ºc ?
Answers: 2

You know the right answer?
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid...
Questions

History, 17.10.2020 08:01


History, 17.10.2020 08:01



Health, 17.10.2020 08:01

Mathematics, 17.10.2020 08:01



Business, 17.10.2020 08:01


Chemistry, 17.10.2020 08:01



Mathematics, 17.10.2020 08:01





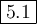
![K_{\text{eq}} = \dfrac{\text{[SO$_{3}$]}^{2}}{\text{[SO}_{2}]^{2}\text{[O$_{2}$]}} = \dfrac{0.0200^{2}}{0.0307^{2}\times0.0833} =\mathbf{5.1}\\\\\text{The value of the equilibrium constant is $\large \boxed{\mathbf{5.1}}$}](/tpl/images/0711/0231/1d368.png)


