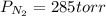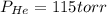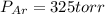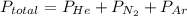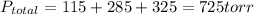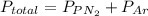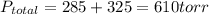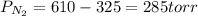A 3.00 L cylinder at 25 degrees Celsius contains a mixture of 3 gases: He, N2, and Ar at partial pressures of 115, 285, and 325 torr, respectively. If all the He is removed from the mixture and the temperature does not change, what will be the partial pressure, in torr, of the N2

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
This question is about electrolysis. metal spoons can be coated with silver. this is called electroplating. suggest one reason why spoons are electroplated?
Answers: 1

Chemistry, 22.06.2019 12:30
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1

Chemistry, 22.06.2019 18:10
Measurements that have similar values are: a. usually accurate b. sometimes accurate c. always accurate d. never accurate
Answers: 1

Chemistry, 22.06.2019 19:50
If a gas has an initial pressure of 101kpa and a volume of 10l, then it expands to a volume of 20l, what is the new pressure?
Answers: 2
You know the right answer?
A 3.00 L cylinder at 25 degrees Celsius contains a mixture of 3 gases: He, N2, and Ar at partial pre...
Questions

Mathematics, 21.08.2019 06:30






History, 21.08.2019 06:30


Biology, 21.08.2019 06:30

Geography, 21.08.2019 06:30

History, 21.08.2019 06:30

Biology, 21.08.2019 06:30

Mathematics, 21.08.2019 06:30


Mathematics, 21.08.2019 06:30

Mathematics, 21.08.2019 06:30

Mathematics, 21.08.2019 06:30