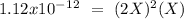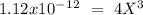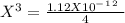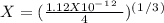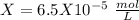Chemistry, 23.07.2020 19:01 dustonangiecook
chromate is sparingly soluble in aqueous solutions. The Ksp of Ag2CrO4 is 1.12×10−12 . What is the solubility (in mol/L) of silver chromate in 1.00 M potassium chromate aqueous solution?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:50
What is the temperature of one mole of helium gas at stp?
Answers: 3


Chemistry, 22.06.2019 12:00
Which of the following units is not an official si unit? mole liter kilogram ampere
Answers: 1

You know the right answer?
chromate is sparingly soluble in aqueous solutions. The Ksp of Ag2CrO4 is 1.12×10−12 . What is the s...
Questions


Mathematics, 13.05.2021 20:40

Biology, 13.05.2021 20:40

Mathematics, 13.05.2021 20:40

Social Studies, 13.05.2021 20:40


Spanish, 13.05.2021 20:40

Mathematics, 13.05.2021 20:40

History, 13.05.2021 20:40


Mathematics, 13.05.2021 20:40

History, 13.05.2021 20:40


Advanced Placement (AP), 13.05.2021 20:40

Mathematics, 13.05.2021 20:40



English, 13.05.2021 20:40


Chemistry, 13.05.2021 20:40

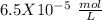
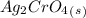 , so:
, so: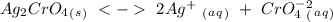
![Kps~=~[Ag^+]^2[CrO_4^-^2]](/tpl/images/0711/8854/b7f4a.png)
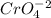 formed, 2 moles of
formed, 2 moles of 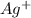 are formed. We can use "X" for the unknown concentration of each ion, so:
are formed. We can use "X" for the unknown concentration of each ion, so:![[CrO_4^-^2]~=~X](/tpl/images/0711/8854/4fd99.png) and
and ![[Ag^+]~=~2X](/tpl/images/0711/8854/da6f8.png)