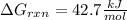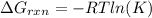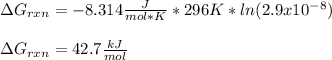Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 05:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3

Chemistry, 22.06.2019 07:30
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.036m naoh best answer will be brainliest
Answers: 3
You know the right answer?
Calculate the value of ΔG∘rxnΔGrxn∘ for the following reaction at 296 K. Ka = 2.9 × 10–8 and assume...
Questions

Mathematics, 19.08.2019 15:00

Biology, 19.08.2019 15:00

History, 19.08.2019 15:00

Mathematics, 19.08.2019 15:00

Mathematics, 19.08.2019 15:00


Mathematics, 19.08.2019 15:00

History, 19.08.2019 15:00


History, 19.08.2019 15:00

Mathematics, 19.08.2019 15:00





Mathematics, 19.08.2019 15:00




Social Studies, 19.08.2019 15:00