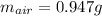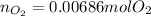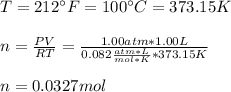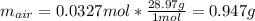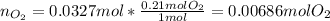Chemistry, 24.07.2020 02:01 YamiletRdz721
Suppose you have a container filled with air at 212 oF. The volume of the container 1.00 L, the pressure of air is 1.00 atm. The molecular composition of air is 79% N2 and 21% O2 for simplification. Calculate the mass of air and moles of O2 in the container.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:10
Agas diffuses 1/7 times faster than hydrogen gas (h2). what is the molar mass of the gas? 100.10 g/mol 98.78 g/mol 86.68 g/mol 79.98 g/mol
Answers: 3

Chemistry, 23.06.2019 02:00
The point along a planet's orbit where it is closest to the sun is called the
Answers: 1

Chemistry, 23.06.2019 06:00
What does it mean for something to be dissolved in watera- it is submerged in water moleculesb-it is stirred in the water moleculesc- it is surrounded by water molecules d-it has water molecules added to it
Answers: 2

Chemistry, 23.06.2019 10:30
Using the periodic table, complete the following. element: hydrogen symbol: h₂ molecular weight: g mass of one mole: g/mol
Answers: 2
You know the right answer?
Suppose you have a container filled with air at 212 oF. The volume of the container 1.00 L, the pres...
Questions


History, 17.12.2019 01:31


Physics, 17.12.2019 01:31

History, 17.12.2019 01:31




History, 17.12.2019 01:31



Social Studies, 17.12.2019 01:31

Mathematics, 17.12.2019 01:31


Social Studies, 17.12.2019 01:31