Question 4
1 points
Save Answer
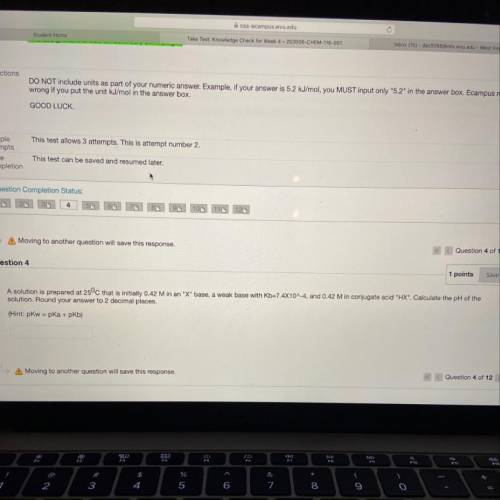
A solution is prepared at 25°C that is initially 0.42 M...

Chemistry, 23.07.2020 06:01 jaylen2559
Question 4
1 points
Save Answer
A solution is prepared at 25°C that is initially 0.42 M in an "X" base, a weak base with Kb=7.4X10^-4, and 0.42 M in conjugate acid "HX". Calculate the pH of the
solution. Round your answer to 2 decimal places.
(Hint: pkw=pka + pkb)
A Moving to another question will save this response.
< Question 4 of 12>

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 12:00
Ineed this asap part i: scientific method what is the difference between science and pseudoscience? what is the scientific method?
Answers: 2

Chemistry, 22.06.2019 16:10
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.300 m , [b] = 1.05 m , and [c] = 0.550 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.140 m and [c] = 0.710 m . calculate the value of the equilibrium constant, kc.
Answers: 1

Chemistry, 22.06.2019 21:50
What is a main difference between a mixture and a pure substance? a mixture is only a liquid, but a pure substance can be in any state.a mixture looks the same throughout, but a pure substance does not.1 a mixture can vary in composition, but a pure substance has a set composlo a mixture can be made up of a single compound, but a pure substance car
Answers: 2
You know the right answer?
Questions



Mathematics, 09.04.2020 22:37

Mathematics, 09.04.2020 22:37


History, 09.04.2020 22:37

History, 09.04.2020 22:37

Spanish, 09.04.2020 22:37

Mathematics, 09.04.2020 22:37

English, 09.04.2020 22:37

Biology, 09.04.2020 22:37





Biology, 09.04.2020 22:37


Biology, 09.04.2020 22:37




