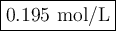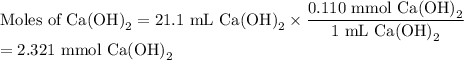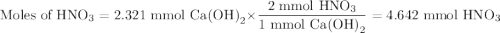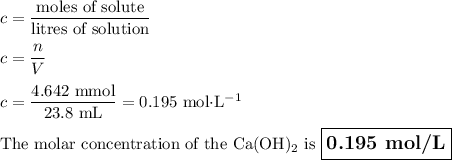Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:30
One mole of zinc has a mass of 65.4 grams. approximately how many atoms of zinc are present in one mole of zinc? 32 × 1023 atoms 6 × 1023 atoms 66 atoms 65 atoms
Answers: 1

Chemistry, 21.06.2019 20:00
Agas in a balloon at constant pressure has a volume of 160 ml at -125*c. what is its volume at 29.0*c?
Answers: 1

Chemistry, 22.06.2019 02:00
Which of the following happens during cell division? (a) energy is created (b) waste is eliminated (c) carbon dioxide is released (d) damaged cells are replaced
Answers: 1

Chemistry, 22.06.2019 19:00
How does kepler second law of planetary motion overthrow one of the basic beliefs of classical astronomy
Answers: 1
You know the right answer?
An aqueous solution of nitric acid is standardized by titration with a 0.110 M solution of calcium h...
Questions

Mathematics, 14.09.2020 04:01

English, 14.09.2020 04:01

Mathematics, 14.09.2020 04:01

Mathematics, 14.09.2020 04:01

Physics, 14.09.2020 04:01

Mathematics, 14.09.2020 04:01

Mathematics, 14.09.2020 04:01

Physics, 14.09.2020 04:01

Mathematics, 14.09.2020 04:01

Mathematics, 14.09.2020 04:01

Mathematics, 14.09.2020 04:01

Mathematics, 14.09.2020 04:01

Mathematics, 14.09.2020 04:01

Social Studies, 14.09.2020 04:01

Mathematics, 14.09.2020 04:01

Health, 14.09.2020 04:01

Mathematics, 14.09.2020 04:01

Mathematics, 14.09.2020 04:01

Mathematics, 14.09.2020 04:01

Social Studies, 14.09.2020 04:01